Table of Contents
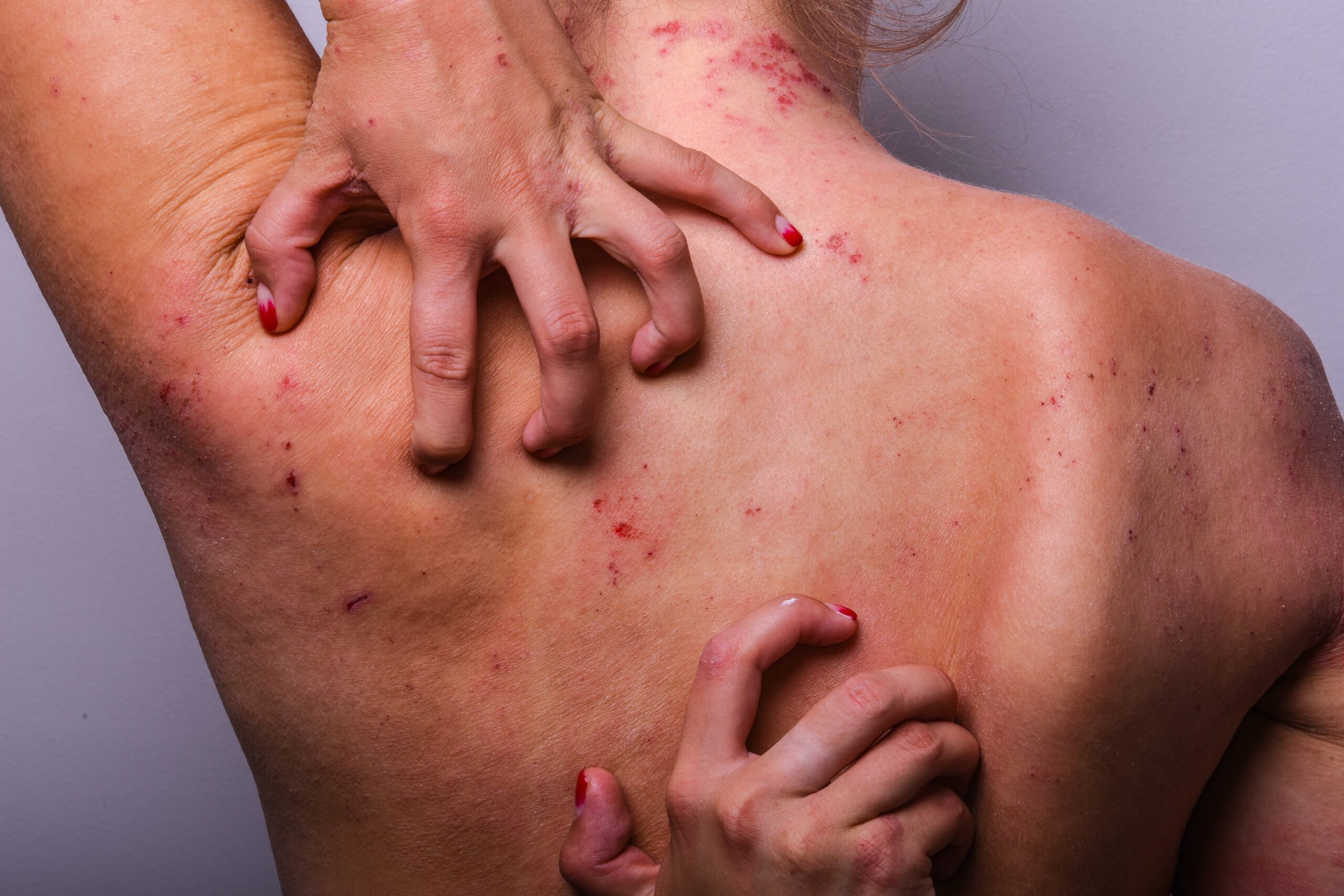
The FDA has issued a warning about severe itching that can occur when patients stop taking common allergy medications cetirizine (Zyrtec) and levocetirizine (Xyzal) after long-term use, highlighting the need for careful discontinuation.
At a Glance
- The FDA has mandated new warnings for cetirizine and levocetirizine after identifying 209 cases of severe itching following discontinuation
- Pruritus (severe itching) typically occurs within days of stopping these medications after months or years of daily use
- Restarting the medication resolved symptoms in 90% of cases, with some patients benefiting from a gradual tapering approach
- Patients should consult healthcare providers before stopping these medications, especially after long-term use
Understanding the Warning
The FDA has recently required a warning about the risk of severe itching (pruritus) after stopping long-term use of oral allergy medications cetirizine and levocetirizine, commonly known by brand names Zyrtec and Xyzal. This warning affects both prescription and over-the-counter versions of these widely used antihistamines, which treat allergic rhinitis and chronic idiopathic urticaria. The mandated label changes come after careful review of reported cases where patients experienced debilitating itching upon discontinuation.
Between April 2017 and July 2023, the FDA identified 209 cases of post-discontinuation pruritus globally, with 197 cases occurring in the United States. The affected individuals had typically used these medications daily for extended periods—months or often years—without experiencing itching problems before starting the medications. The severe itching generally began within days of stopping the medication.
Severity and Impact
Although rare, some cases were serious enough to require medical intervention. The itching significantly impacted patients' quality of life, with some cases leading to disability, hospitalization, and in extreme instances, thoughts of suicide or self-harm. This underscores the importance of recognizing and addressing this potential side effect promptly. The FDA noted that while the exact mechanism causing the withdrawal-related pruritus remains unknown, their evaluation supports a causal relationship between stopping these medications and the onset of severe itching.
The Consumer Healthcare Products Association has expressed support for the FDA's commitment to notifying the public about safety signals, even when extremely rare. They emphasized that consumer safety remains the top priority for manufacturers of these medications. Healthcare professionals are now advised to discuss the risk of pruritus with patients when prescribing or recommending these medicines, especially when intended for chronic use.
Managing Discontinuation
For patients experiencing severe itching after stopping cetirizine or levocetirizine, the FDA noted that restarting the medication resolved symptoms in approximately 90% of cases. Some patients benefited from tapering off the medication after restarting it, suggesting that gradual discontinuation may be preferable to abrupt cessation. This finding is particularly important for long-term users who may be considering stopping their allergy medication.
Healthcare providers are encouraged to provide individualized guidance for patients on long-term cetirizine or levocetirizine therapy. The FDA specifically recommends that patients contact their healthcare provider if they experience severe itching after stopping either medication. Consumers are strongly advised not to discontinue these medications abruptly without first consulting their healthcare provider, especially if they've been taking them regularly for an extended period.
Moving Forward
The FDA continues to monitor the situation and will provide updates as more information becomes available. They've updated prescribing information to include warnings about pruritus risk and are encouraging both patients and healthcare providers to report adverse events to the FDA's MedWatch program. While these medications remain effective treatments for allergy symptoms, this new warning emphasizes the importance of proper medication management, particularly when discontinuing drugs after prolonged use.
Beyond the newly identified risk of post-discontinuation pruritus, patients should be aware that cetirizine and levocetirizine can cause other side effects, including fatigue, drowsiness, dry mouth, and gastrointestinal symptoms. For most patients with seasonal or occasional allergies who use these medications intermittently, the risk of withdrawal itching is minimal. However, those who rely on daily, long-term use should work closely with their healthcare providers to develop appropriate treatment plans that include strategies for safe discontinuation when necessary.
AD
Most Recent
AD
